Synthetic Gasoline
Latest update on 15 April 2023
Synthetic liquid fuels are any alternative method of producing liquid fuel to extracting and refining crude. Synthetic gasoline is a synthetic fuel mainly made of C6-C10 hydrocarbons to match gasoline properties. There are numerous competing methods to produce synthetic gasoline:
- Fischer-Tropsch process
- Methanol-to-Gasoline (MTG)
- Syngas-to-Gasoline (STG+)
- Microbial isobutene, upgraded to branched alkanes
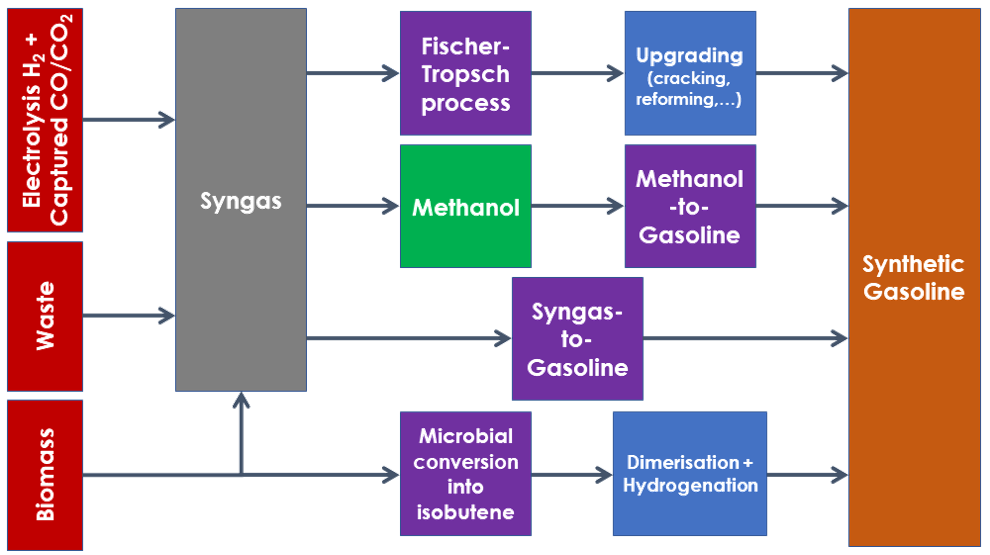
Main processes to produce synthetic gasoline
All first 3 methods use syngas (synthetic gas) as an intermediate product. Syngas is a mixture of hydrogen and carbon monoxide or dioxide, sometimes diluted by traces of other reactive or inert gases, that can be created based on a wide range of feedstock.
Synthetic Gasoline vs eGasoline
eFuels are a subcategory of synthetic fuels using electricity as power source
- To capture CO or CO2 as a source of carbon atoms
- To electrolyse water into H2 as a source of hydrogen atoms
- To power pumps and heaters feeding the chemical reactors
The syngas (synthetic gas) thereby produced is conceptually identical to coal-derived, natural-gas-derived, waste-derived or biomass-derived syngas, so that the reminder of the synthetic fuel production is identical to any other source of syngas. The only potential difference is the relative content in H2, CO, and CO2.
Process comparison
The Fischer-Tropsch process is historically the first developed and the only one to have reached mass production mainly in Germany and in South Africa, in both cases exploiting coal availability to compensate for crude embargoes. However, because it relied on syngas (obtained through coal gasification), it can be adapted to almost any source of syngas (natural gas, waste, biomass, captured CO/CO2 and electrolysis H2). Another key specificity of the Fischer-Tropsch process is the wide range of molecules produced (from methane to waxes), so that it can produce synthetic fuels compatible with gasoline, jet fuel, and Diesel. This is also a drawback as the wide range requires additional refining/upgrading steps when targeting a specific output.
The Methanol-to-Gasoline and Syngas-to-Gasoline processes are very similar, converting syngas into methanol into DME and finally into C4-C10 gasoline-like molecules, with differences mainly in integration between steps and therefore process efficiency. Produced molecules are mainly branched alkanes, cycloalkanes and aromatics. Conventional refinery processes can be added to the strict MTG/STG+ processes to optimise the end-product properties.
The isobutene to branched alkane process is the only process not linked to syngas, but rather to a biological conversion of biomass into isobutene, followed by dimerization (combination of 2 identical molecules) into iso-octene and hydrogenation into iso-octane. This process therefore mostly produces a pure compound synthetic fuel with high octane rating.
