Ammonia
Published on 22 January 2023
Ammonia is a carbon-free compound, mostly used as a fertilizer or a refrigerant, but that can also be used as a fuel or an energy carrier thanks to its large content in hydrogen: ammonia (NH3) is made of 1 atom of nitrogen and 3 atoms of hydrogen.
At ambient conditions, it is a stable, colourless gas, but unlike hydrogen it only needs to be cooled down to -33°C or pressurised up to 10 bar to turn into a liquid.
Ammonia is naturally occurring as a biological waste and widely used as a fertiliser. It has a pungent smell, helping for leak detection, but is caustic and hasardous in pure form (rarely pure in nature). Managing toxicity would notably be a challenge if ammonia were to be used as an everyday fuel.
Ammonia is abundant and already produced in large quantities as a fertiliser, as a building block for numerous pharmaceutical applications or as a cleaning product. Yet, its use as a fuel is currently anecdotical.
How to produce Ammonia?
Ammonia is produced by combining nitrogen from air and hydrogen. However, these only combine at high pressure and high temperature, and a catalytic process (generally the Haber-Bosch process) is required for industrial production.
As nitrogen comes from air, the energy consumption and pollution to produce ammonia mainly relate to the production of hydrogen, as well as reaching high pressure and temperature to sustain the catalytic combination.
Nowadays, most ammonia is produced with hydrogen derived from methane, through steam reforming. With this method, worldwide ammonia production for fertiliser use only accounts for 1.2% of all greenhouse gas emissions, so that alternative methods for ammonia production are required.
If produced based on methane, ammonia would be a carbon-free fuel, but with a heavy equivalent CO2 content linked to the production steps. To make it a truly environmentally-friendly fuel, it should only be produced based on green hydrogen (hydrogen derived from renewable resources). Note that this would lead to a low equivalent CO2 content, but not to a zero equivalent CO2 content as even renewable resources have equivalent greenhouse gas emissions.
Ammonia properties as a fuel
Ammonia is not prone to auto-ignition, so that it is not a good candidate to be used in compression-ignited internal combustion engines.
However, ammonia also has a very slow flame propagation speed (~8 cm/s at ambient conditions vs ~36 cm/s for gasoline), so that it is not very well suited to spark-ignited internal combustion engines either.
Ammonia has a low energy content of about 18.6 MJ/kg, i.e. ~2.25 times lower than gasoline and similar to methanol. However, it has an air-fuel ratio of only ~6 (compared to ~14.5 for gasoline), so that more fuel can be added by amount of air in an engine (air flow is often limiting engine power) by a ratio of ~2.4, more than compensating the gap in energy content. Yet, the slow burning nature restricts efficiency and effective power output.
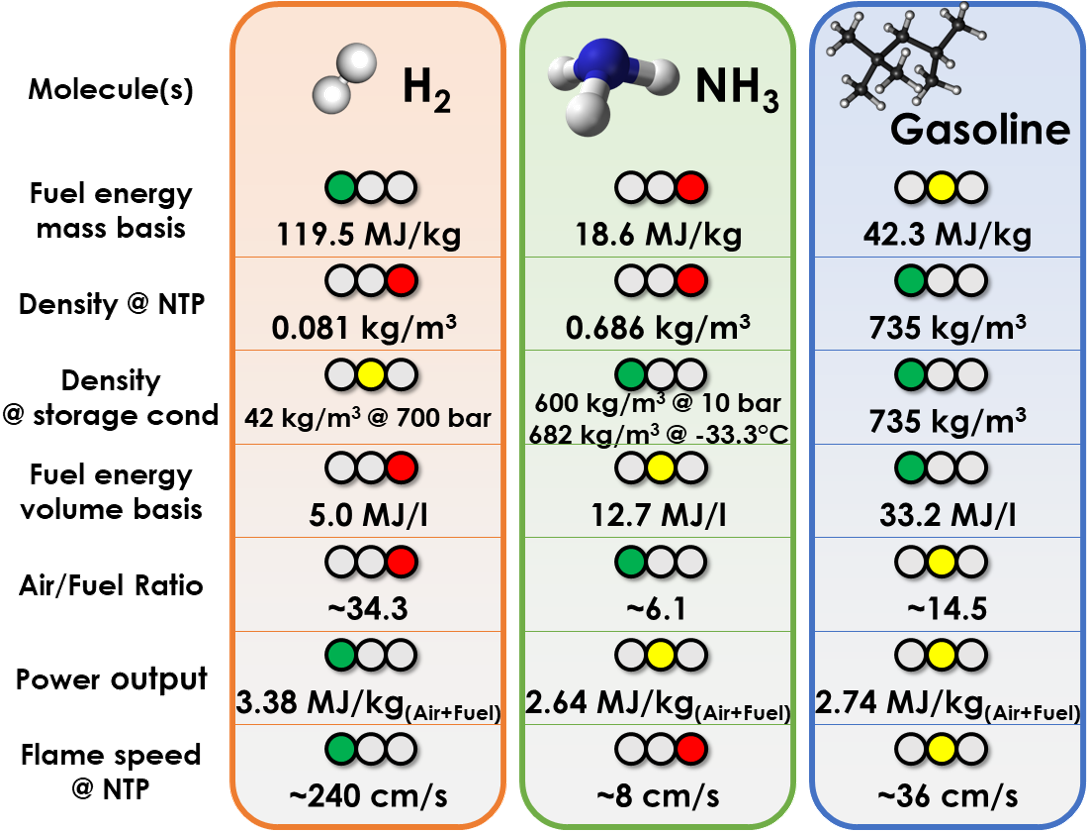
Comparison of properties of ammonia, hydrogen and gasoline
Because it is a polar molecule, ammonia in liquid form is very stable and it has a large latent heat of vapourisation of ~1375 kJ/kg, i.e. more than 4 times gasoline and more than alcohol fuels (+15% on methanol, +50% on ethanol). With similar air-fuel ratio and similar heat of vapourisation as methanol, a similar very large cooling of the intake charge by fuel evaporation can be expected, which is beneficial to air breathing. Yet, thanks to its gaseous nature at ambient conditions, evaporation should be easier, while methanol can suffer from incomplete evaporation because of excessive air/fuel mixture cooling.
Which use case for ammonia as a fuel?
Because ammonia burns very slowly, it is better suited to slowly rotating engines.
Because of its toxicity and related handling requirements, it is better suited for a use in a professional environment.
Because storage as a liquid requires cooled or well-insulated tanks, usage as a replacement to LNG/LPG applications already having such tanks is a natural fit.
For all these reasons, the long-distance shipping industry is the primary target for ammonia introduction as a fuel. Numerous container ships or supertankers already operate on liquefied gas and engines typical have a nominal speed of 100 RPM.
